Medicine Verification
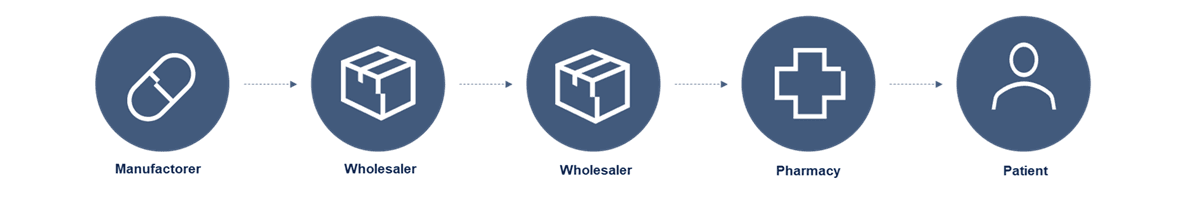
Before a medicine can be distributed to patients in Denmark and the rest of the EU, it must first be approved by the authorities, typically the European Medicines Agency (EMA) or the Danish Medicines Agency. Once the approval is in place, both originator and generic manufacturers can manufacture the medicine under strict quality requirements.
After production, the medicines are handed over to approved wholesalers, who are responsible for storing and distributing the medicines safely to pharmacies and hospitals. The wholesalers ensure that the supply chain is unbroken and that the medicine is handled correctly all the way to delivery.
Parallel importers also play a role in the supply chain. They buy authorised medicines in one EU country, repackage them according to national requirements and resell them in another EU country, where the price may be higher. This contributes to security of supply and competition in the markets.
The collaboration between the Danish Medicines Agency, pharmaceutical manufacturers and parallel importers, wholesalers, pharmacies, hospitals and DMVO (Danish Medicine Verification Organisation) is crucial to ensure that only approved and controlled medicines reach patients. Systems such as DMVS and EMVS ensure that the medication can be tracked and verified, protecting against counterfeit medicines.
Do you need help?
For inquiries related to e.g., alert investigation, the development of the DMVS system, contract details, or upcoming updates, please do not hesitate to contact us at info@dmvo.dk or call +45 39 27 60 60.
